Unmet Need
Problem statement
- The current ICH regulatory guidances for CSRs are the 1995 ICH E3 and the 2012 supplementary Q & A. The Q & A addresses some of the ambiguity inherent to ICH E3.
- All CSRs submitted in MAAs to EMA will be publicly disclosed. The associated risks are largely privacy-related. Minimising the risks is paramount.
Contribution of the CORE Reference project
CORE Reference is a user manual to help medical writers navigate relevant (ICH, and EU and US regional) guidelines as they create CSR content for today’s studies, including detailed content suggestions and practical suggestions for developing CSRs that will require minimum redaction and modification prior to public disclosure.
Abbreviations: CSR, clinical study report; EMA, European Medicines Agency; ICH, International Council for Harmonisation; MAA, marketing authorisation application; Q & A, Questions and Answers.
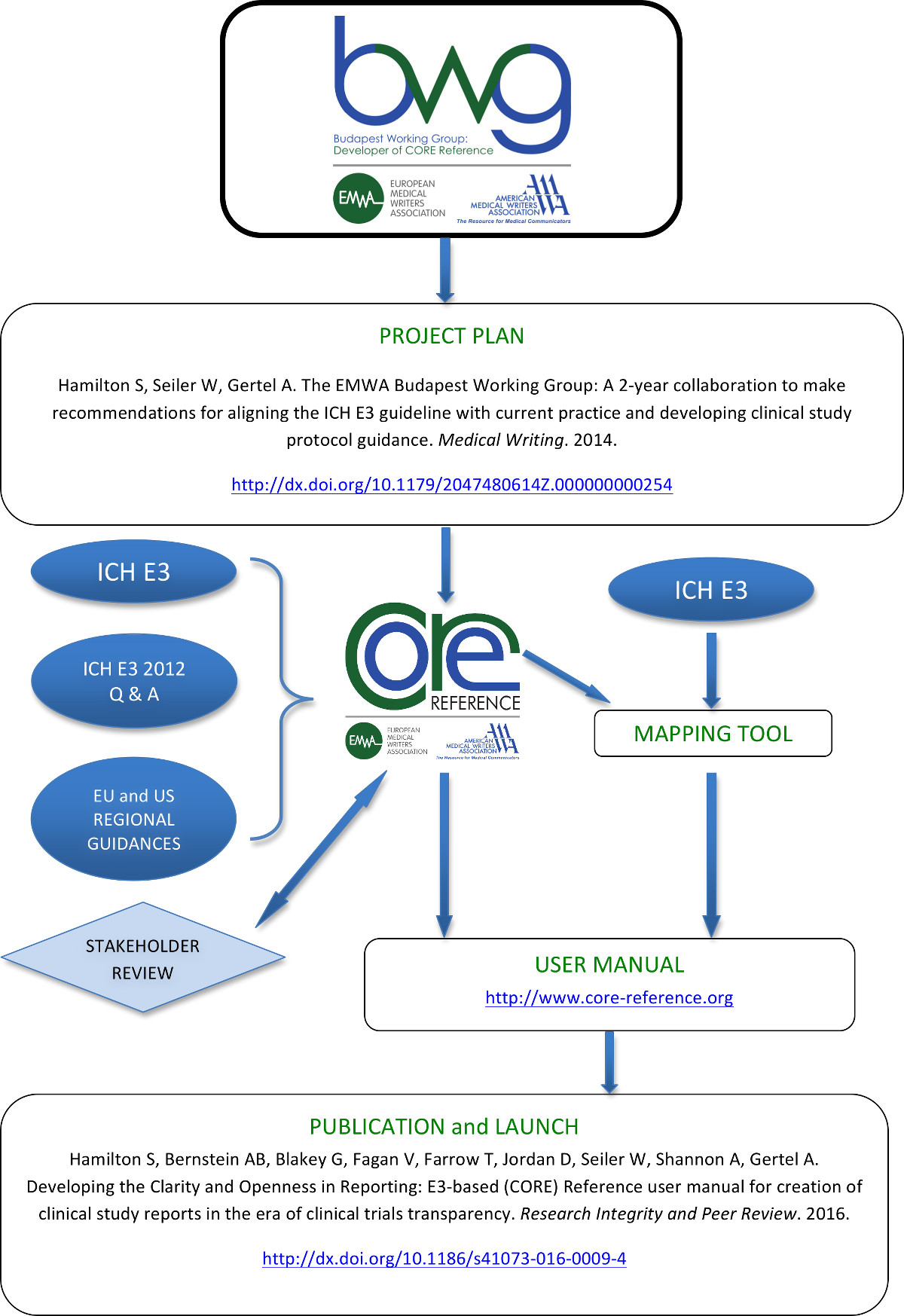
Process map of the CORE Reference project
Reproduced from the original publication under a Creative Commons license (http://creativecommons.org/licenses/by/4.0/)
http://researchintegrityjournal.biomedcentral.com/articles/10.1186/s41073-016-0009-4 and
http://dx.doi.org/10.1186/s41073-016-0009-4
Stakeholders: Stakeholders contributing to the development of CORE Reference included: Health Canada; Drug Information Association (DIA) Medical Writing Community’s CORE Review Task Force; Academic and Principal Investigator, Todd E. Pesavento, MD and Patient Advocate, David Gilbert.
Abbreviations: AMWA, American Medical Writers Association; BWG, Budapest Working group; CORE Reference, Clarity and Openness in Reporting: E3-based; EMWA, European Medical Writers Association; ICH, International Council for Harmonisation; ICH E3, ICH Harmonised Tripartite Guideline: Structure and Content of Clinical Study Reports E3. Step 4. 30 Nov 1995; ICH E3 2012 Q & A, ICH E3 Guideline: Structure and Content of Clinical Study Reports Questions & Answers (R1). 6 July 2012.
From 2025: Follow CORE Reference on LinkedIn.